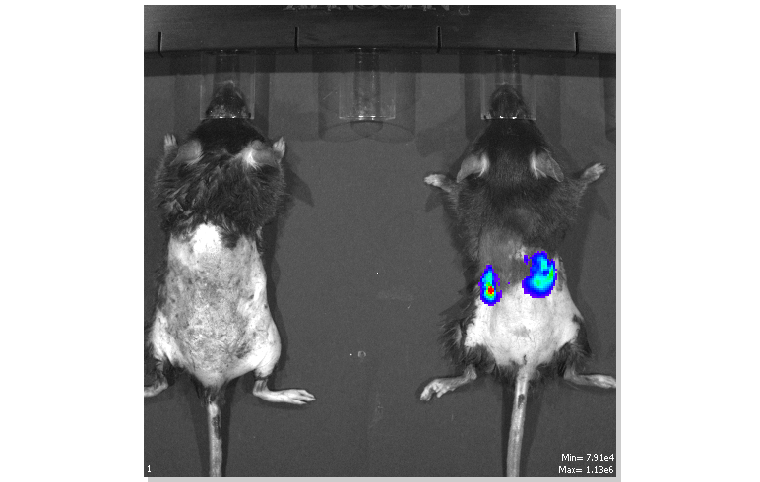
NLAC established the "Platform for Next-generation Nephrotoxicity Testing in Mice" (also known as the "Shennong Mouse Nephrotoxicity Screening Platform") to supply much-needed lab mice to domestic developments in nephrotoxicity testing and kidney treatment products, while also reducing the possibility of nephrotoxicity-inducing substances from affecting the citizenry.
Currently, global markets lack truly effective testing methods for the renal toxicity of drugs, health foods or food additives. NLAC's Shennong Mouse Nephrotoxicity Screening Platform binds the kidney-specific enzyme myo-inositol oxygenase (MIOX) with foreign luminescence enzymes, and when the kidney is damaged, the organ's cells will release this luminescent enzyme into the blood or urine. Through measuring the activity of luminescence enzyme in the blood or urine of the lab mice, the degree of kidney damage can then be assessed. This method is both more economical and effective than the analysis method of rat urine kidney damage protein jointly developed by the "Predictive Safety Testing Consortium", which had been established in 2005 by major international pharmaceutical companies and the public drug management agencies of the US, EU and Japan.